T. E. Weber, A. P. Schinckel, and M. E. Spurlock
Department of Animal Sciences
Introduction
The activation of the innate immune system in growing pigs via peripheral injection of lipopolysaccharide (LPS) induces a pronounced inflammatory response and alters metabolism. This makes the use of LPS injection a valuable model in the study of the mechanisms of the inflammatory response. The inflammatory response is believed to be mediated via the action of proinflammatory cytokines such as tumor necrosis factor a (TNF-a) which are released by antigen presentation cells upon encountering LPS. Indeed, several investigators have demonstrated that peripheral injection of LPS increases circulating concentrations of TNF-a (Leininger et al., 2000; Webel et al., 1997; Wright et al., 2000). Additionally, peripheral LPS challenge has been shown to decrease circulating concentrations of IGF-I in growing pigs (Wright et al., 2000). However, genetic population differences for immunological and somatotropic responses to peripheral LPS have not been investigated.
It has been demonstrated that certain genetic populations of pigs are more sensitive to immunological stressors when reared in sub-optimal environments. Pigs of a high lean genotype experience reduced growth performance and increased mortality rates as compared to a lower lean genotype when reared in facilities managed under a conventional weaning, continuous flow system (Frank et al., 1997; 1998). Furthermore, the mechanisms responsible for this increased environmental sensitivity have not been delineated. Peripheral injection of LPS may serve as a model to evaluate mechanisms responsible for genetic differences in sensitivity to environmental stressors.
Our objective was to determine whether there were differences in the immunological and somatotropic responses to LPS in growing pigs belonging to different genetic populations.
Materials and Methods
Animals and experimental design. Female pigs of two genetic populations (European terminal cross and American terminal cross; n = 20) were randomly assigned (stratified by body weight; BW) to one of two LPS injection treatments. The LPS treatment consisted of either 11.4 mg/lb BW E.coli LPS (LPS) given i.m. or an equivalent volume of sterile saline (SAL). The average BW for the European terminal cross (ET) and American terminal cross (AT) gilts were (mean ± SD) 112 ± 7.5 lb and 115 ± 4.8 lb, respectively. The pigs were housed in individual pens, had free access to feed and water, and were allowed an acclimation period of 5-d prior to the initiation of the LPS treatments. On the day that the LPS treatments were imposed, access to feed was denied for a period of 12 h prior to and during the LPS challenge in order to remove any confounding effects of feed intake on physiological and immunogical parameters measured. Separate groups of these two genetic lines of pigs were raised to market weight and final body weight, backfat, and longissimus muscle depth were measured. The animal handling protocols were approved by the Purdue University Animal Care and Use Committee.
Serum and tissue sample collection. Blood samples were collected via jugular venipuncture at 0, 2, and 4 hours relative to LPS or saline injection. Serum samples were divided into 1 mL aliquots and stored at -80°C until analysis. Rectal temperatures were measured with a digital thermometer at 0 and 2 hours relative to LPS treatments. Pigs were killed by electrocution followed by exsanguination 4 hours after the initiation of the LPS treatments. At this time, samples of liver and longissimus muscle were quickly dissected, frozen in liquid nitrogen, and stored at –80°C until extraction for total RNA.
Serum and RNA analyses. Serum IGF-I and GH concentrations were quantified via RIA procedures validated previously (Taylor-Roth et al., 1998). The intrassay CV’s for the IGF-I and GH assays were 11.2% and 13.0%, respectively. Serum cortisol and TNF-a concentrations were determined via commercially available ELISA kits. Intra- and interassay CV’s were less than 10%. Total RNA was extracted from liver samples and the abundance of IGF-I mRNA was determined via a ribonuclease protection assay.
Statistical analysis. The data for carcass characteristics were analyzed by t-test. All other data were analyzed by ANOVA as a 2 x 2 factorial arrangement of treatments. The ANOVA was performed using the GLM procedure of SAS (SAS Inst., Cary, NC). The main effects of genetic population and LPS treatments as well as the interaction between genetic population and LPS were included in the model. When the interaction was found to be significant, means were separated using the least significant difference test. Serum variables were analyzed as repeated measures.
Results
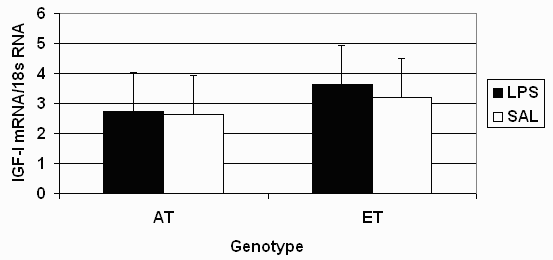
Injection with LPS decreased (P = 0.04) circulating concentrations of IGF-I at 2 and 4 hours post injection (Table 2). However, there was no effect of genotype or LPS on liver IGF-I mRNA at 4 hours after LPS injections (Figure 1). There was an interaction (P = 0.01) between genotype and LPS regimen for serum growth hormone (GH) at 2 hours post injection. Growth hormone levels were decreased (P < 0.05) in AT gilts, whereas LPS had no effect on serum GH in ET gilts. An interaction occurred (P = 0.01) for serum TNF-a at 2 and 4 hours post LPS, due to ET gilts having a three-fold greater increase (P < 0.01) of TNF-a in response to LPS than AT gilts. Injection with LPS increased serum cortisol levels in both genetic populations at 2 hours (P < 0.01) and 4 hours (P < 0.10) post-injection. Rectal temperatures were increased (P < 0.01) by LPS in both genetic populations, but tended (P = 0.10) to be more dramatically increased in ET gilts than for AT gilts at 2 hours after the LPS injections.
Discussion
The results of this experiment demonstrate that genetic populations of pigs may respond differently to immunological challenges. The leaner (Table 1) European terminal cross gilts had higher concentrations of TNF-a and body temperatures in response to LPS than did the American terminal cross gilts. More interesting is that the differences in leanness characteristics, while significant, were fairly small between the two genetic populations in this study. This indicates that selection for leanness, even though differences in leanness may be small, has changed some aspects of the innate immune system and may be the reason that certain genetic populations of pigs respond more unfavorably than others to sub-optimal rearing environments and pathogen/antigen exposure (Frank et al., 1997, 1998; Leininger et al., 2000).
As in other experiments (Spurlock et al., 1998; Wright et al., 2000), treatment with LPS decreased serum concentrations of IGF-I. However, liver IGF-I mRNA levels were not affected by LPS injection. This suggests other mechanisms than transcriptional control in the liver for the decrease in serum IGF-I concentrations in response to LPS injections. Therefore, further research is warranted to examine mechanisms by which certain genetic populations of pigs are more sensitive to LPS and how LPS decreases circulating IGF-I concentrations.
Implications
This research demonstrates that there are genotypic differences in how pigs respond to innate immune system stimulation with LPS. Additionally, this research points to other mechanisms via which circulating concentrations of IGF-I are decreased following immunological challenge.
Literature Cited
Frank, J. W., B. T. Richert, A. P. Schinckel, B. A. Belstra, and A. L.Grant. 1997. Environmental effects on genetic potential for lean gain. J. Anim. Sci. 75(suppl. 1):38.
Frank, J. W., B. T. Richert, A. P. Schinckel, B. A. Belstra, S. F. Amass, and S. A. DeCamp. 1998. Effects of environment, genotype, and health management system on pig growth performance and carcass characteristics. Purdue University Swine Day Report. 1998:129-140.
Leininger, M. T., C. P. Portocarrero, A. P. Schinckel, M. E. Spurlock, C. A. Bidwell, J. N. Nielsen, and K. L. Houseknecht. 2000. Physiological response to acute endotoxemia in swine: effect of genotype on energy metabolites and leptin. Domest. Anim. Endocrinol. 18:71-82.
Spurlock, M. E., M. A. Ranalletta, S. G. Cornelius, G. R. Frank, G. M. Willis, S. Ji, A. L. Grant, and C. A. Bidwell. 1998. Leptin expression in porcine adipose tissue is not increased by endotoxin but is reduced by growth hormone. Journal of Interferon and Cytokine Research. 18:1051-1058.
Taylor-Roth, J. L., P. V. Malven, D. E. Gerrard, S. E. Mills, and A. L. Grant. 1998. Independent effects of food intake and insulin status on insulin-like growth factor-I in young pigs. Comp. Biochem. Physiol. 120:357-363.
Webel, D. M., B. N. Finck, D. H. Baker, and R. W. Johnson. 1997. Time course of increased plasma cytokines, cortisol and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75:1514-1520.
Wright, K. J., R. Balaji, C. M. Hill, S. S. Dritz, E. L. Knoppel, and J. E. Minton. 2000. Integrated adrenal, somatotropic, and immune responses of growing pigs to treatment with lipopolysaccharide. J. Anim. Sci. 78:1892-1899.
Table 1. Carcass leanness characteristics representative of the two genetic populations a
|
Item |
AT |
ET |
SE |
P-value |
|
Fat depth, in |
0.62 |
0.54 |
0.02 |
0.0001 |
|
Loin depth, in |
2.64 |
2.68 |
0.05 |
0.88 |
|
Lean percentage, % |
55.72 |
56.24 |
0.28 |
0.01 |
a Carcass data representing the two genetic populations of pigs, American terminal cross (AT) or European terminal cross (ET), was collected at a commercial pig processing facility.
Table 2. Effect of genotype and LPS on serum variables and body temperaturea
|
AT |
ET |
P-value |
||||||
|
Item |
LPS |
SAL |
LPS |
SAL |
SEM |
Genotype |
LPS |
Genotype x LPS |
|
|
||||||||
|
0 h |
198.08 |
155.05 |
139.91 |
157.28 |
22.9 |
0.24 |
0.58 |
0.21 |
|
2 h |
115.69 |
128.50 |
68.78 |
141.09 |
19.5 |
0.39 |
0.04 |
0.15 |
|
4 h |
91.83 |
146.63 |
87.30 |
122.33 |
16.3 |
0.39 |
0.01 |
0.55 |
|
|
||||||||
|
0 h |
7.13 |
7.06 |
4.58 |
5.45 |
1.4 |
0.18 |
0.79 |
0.76 |
|
2 h |
3.12 |
9.91 |
3.28 |
4.12 |
1.0 |
0.01 |
0.01 |
0.01 |
|
4 h |
8.53 |
7.82 |
5.99 |
5.30 |
1.9 |
0.22 |
0.73 |
0.99 |
|
|
||||||||
|
0 h |
13.15 |
13.27 |
13.65 |
39.06 |
12.6 |
0.31 |
0.33 |
0.33 |
|
2 h |
464.85 |
11.79 |
1731.80 |
31.03 |
72.4 |
0.01 |
0.01 |
0.01 |
|
4 h |
42.46 |
11.11 |
176.57 |
37.80 |
17.8 |
0.01 |
0.01 |
0.01 |
|
|
||||||||
|
0 h |
6.28 |
7.28 |
6.51 |
4.18 |
1.1 |
0.23 |
0.57 |
0.17 |
|
2 h |
15.53 |
4.21 |
15.70 |
6.11 |
1.3 |
0.45 |
0.01 |
0.53 |
|
4 h |
21.71 |
10.99 |
20.65 |
12.27 |
5.5 |
0.98 |
0.10 |
0.83 |
|
|
||||||||
|
0 h |
101.78 |
102.14 |
102.22 |
102.14 |
0.2 |
0.22 |
0.43 |
0.22 |
|
2 h |
104.26 |
102.18 |
105.18 |
102.06 |
0.3 |
0.21 |
0.01 |
0.10 |
a Gilts (n = 20) of two genetic populations, American terminal cross (AT) or European terminal cross (ET), were injected with 11.4 mg/lb BW E. coli lipolysaccharide (LPS) or an equivalent volume of sterile saline (SAL).

Figure 1. Effect of E. coli lipolysaccharide (LPS) injection and genetic population on liver IGF-I mRNA four hours post LPS injection.