Purdue University 1997 Swine Day Report
J.D. Bishop, G.D. Weesner, P.V. Malven, and W.L. Singleton
Department of Animal Sciences and USDA-ARS
With the advent of increased public awareness and appreciation for animal well-being in livestock production, it is becoming necessary to understand more specifically how animals perceive different situations and assess whether or not their well-being is being met. This is necessary to maintain a level of public respect for current production practices and to enhance the image of livestock production in the United States and worldwide. Through greater scientific understanding of the animal's perception of environmental stimuli and of animal cognition, production facilities and management practices could be altered to meet the needs of the animal, and thereby enhance well-being. In order to fully understand the status of well-being in an animal, we must first identify some indicators of well-being in that animal. Because we are unable to do direct evaluation of any mental sufferings that might be present in the animal, we are forced to utilize other indicators such as physiological, immunological, behavioral, and anatomical indicators of stress and distress. Most researchers agree that low levels of stress are beneficial for animals because they allow them to maintain a homeostatic balance of their physiological systems. It is only when the perception of stressors becomes so great that it affects normal functioning and production of the animal that we utilize the term distress. Stress cannot simply be measured by a single variable but instead must be evaluated based upon several criteria, which will provide information to distinguish the "good stress" from the "bad stress." The objective of this experiment was to gain a greater understanding of how boars physiologically respond to different, and presumably stressful, situations. Specifically, it was hoped to discover a measurable difference between stressful experiences which could be described as being a "good stress" and those which could be described as being a "bad stress."
Eleven purebred boars (10 Yorkshire and 1 Hampshire) ranging in age from 5.5 months to 6 months were trained as if they were to be used for semen collection for artificial insemination (AI) practices. This training involved exposure to a stationary AI dummy sow which the boar mounted. Upon successful mounting of the dummy, semen was collected via the gloved-hand method. After several successful training sessions, the boars were considered fully trained and were placed onto the study. Animals were fitted with indwelling jugular catheters to facilitate frequent blood sampling without disrupting the normal patterns of behavior.
Two treatments were imposed upon the animals during the morning hours: 1) a control (CTRL) treatment which allowed the animal to proceed with normal copulatory behaviors culminating in ejaculation, and 2) a frustration (FRUS) treatment which allowed the animal to mount and display courtship behaviors toward the dummy but no manual pressure was applied to the penis, thus preventing ejaculation. Pre-exposure blood samples were drawn at 5 minute intervals for 30 minutes prior to treatment to establish resting or basal levels of the hormones. Immediately after the final pre-exposure sample, the boar was walked to the nearby collection pen containing the AI dummy and allowed to mount, and if appropriate, semen was collected. During this exposure period, blood samples were collected at the rate of one per minute for the duration of mating or frustration behaviors. Following treatment, blood samples were collected again every 5 minutes for 30 minutes.
Blood samples were analyzed for the following stress-related hormones: cortisol, b-endorphin (b-E), and testosterone. Statistical analysis compared the pre-exposure values to each of the treatments individually as well as comparing the two treatments against each other.
Behaviors were recorded with overhead video cameras for 4 hours after treatment. Behaviors were then divided into active behaviors and passive behaviors. Active behaviors included activities such as walking, standing, eating, or drinking, while passive behaviors consisted of lying down in the housing pen. The video tapes were analyzed to determine the percent of time the animals spent lying down versus time spend engaging in other, more active behaviors.
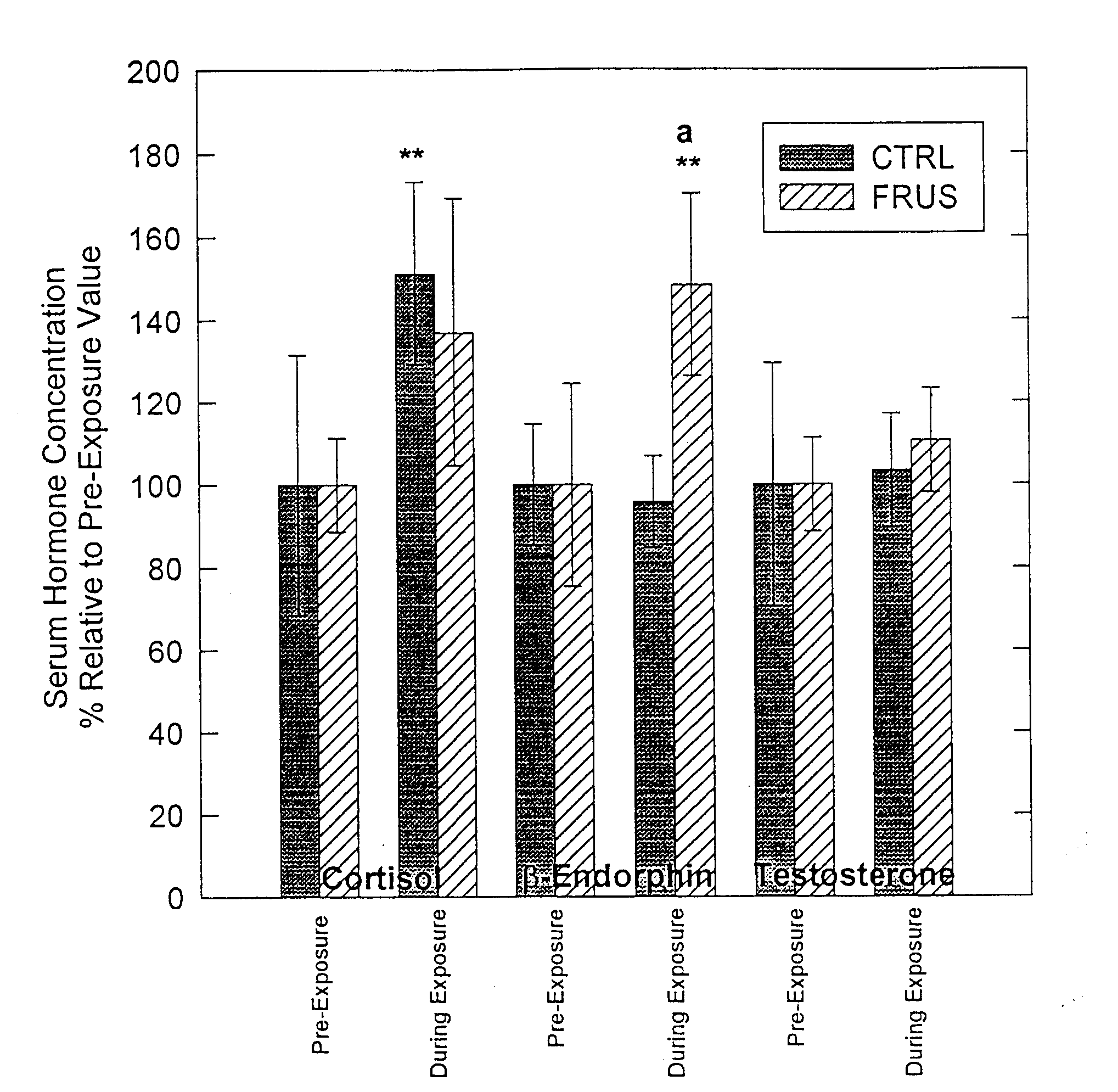
Testosterone levels were similar between treatments, both during and following exposure, while serum cortisol increased during the CTRL (P<.05). Additionally, b-E levels during exposure increased (P<.05) during FRUS treatment but did not increase during CTRL treatment (Figure 1). Finally, following return to the housing pen, cortisol levels were elevated over pre-exposure concentrations for both treatments (CTRL, P<.04; FRUS, P<.06), but no change in other hormone levels were observed (Figure 2).
Analysis of the videotaped behavior indicated that the boars spent less time lying down and more time moving around the pen (P<.05) following a FRUS treatment than when the animal received the CTRL treatment. However, the increased activity of animals receiving the FRUS treatment did not result in more time spent at the available feeding pan.
It is well documented that animals respond to stressors with a variety of physiological responses, and probably the most well-studied is the response of cortisol. Several studies have shown that pigs secrete cortisol in response to a variety of imposed stressors such as confinement, electrical shocks, increased ambient temperature, suckling and weaning, restraint, transportation and sexual arousal. Therefore, the present experiment can be considered a valid model for studying stress because a significant cortisol increase followed both treatments.
Assuming that the model did in fact elicit a stress response in the animal under both treatments, it must then be determined if the animal responded to a seemingly pleasurable but yet stressful situation (CTRL treatment) any differently than to a seemingly frustrating and distressing situation (FRUS treatment).
Testosterone has been correlated with aggression or frustration behaviors in some male animals. It was hoped that testosterone would show some difference between the two treatments in this experiment because the animals were presumably more distressed following the FRUS treatment. However, this was not the case because testosterone failed to show any consistent response to either treatment.
A difference was detected in b-E response to the different treatments. During the period of exposure to the AI dummy in the FRUS treatment, b-E concentrations were elevated over those of the pre-exposure period as well as over their concurrent concentrations during the CTRL treatment. This finding may be related to the occurrence of stress-induced analgesia (SIA), which has previously been reported in some animals such as mice (Morley et al., 1985) and pigs (Dantzer et al., 1986). Animals devote a large portion of their lives to minimizing the effects of stress and pain. Therefore, it would seem logical that mechanisms exist in the body to minimize the effects of stressors. It has previously been reported that endogenous opioid peptides (the class of molecules to which b-E belongs) are thought to participate in the SIA response. It was observed that the b-E values increased in response to the FRUS treatment which prevented a behavior normally reinforced with a positive reward of sexual pleasure. However, similar elevation of b-E was not observed during the CTRL treatment, perhaps suggesting that b-E somehow minimized the effects of the frustration stress presumably sensed by the animal. SIA has been observed in other studies after procedures or manipulations that have aversive or distressful effects upon the animal. However, in some instances, a response similar to SIA has been reported in procedures not thought to be distressing to the animal (McCarthy et al., 1993; Nappi et al., 1982; and Malizia et al., 1979), suggesting that the stressor need not be very severe or even aversive to induce analgesia. A specific role for b-E in producing SIA has also been suggested by the absence of SIA in transgenic mice that cannot produce any b-E because of a genetic defect (Rubinstein et al., 1996). Presumably, the FRUS treatments elicited a form of distress in the animal which is not desirable for maximum production. Behaviorally, the results coincide with the belief that when animals are stressed, or when well-being is not being maximized, they spend less time in productive functions such as resting or eating. This increased activity may negatively affect production of the animals, thereby decreasing profitability of the operation.
The present study may be a scientific approach to distinguish between the different types of environmental stressors perceived by an animal. All stressors cannot be removed from an animal's environment, nor would that be advantageous. However, if we can more effectively determine when and if a stressor actually becomes distressing to the animal, the well-being of the animal may be enhanced. The benefits from a higher level of well-being in the animal are two-fold: 1) increased production from that individual animal because less of its energy and resources are used to counterbalance the distressing event, and 2) increased public acceptance of animal production practices. Both of these can provide an economic reward for the producer.
Dantzer, R., R. Bluthe, and A. Tazi. 1986. Stress-induced analgesia in pigs. Ann. Rech. Vet. 17:147-151.
Malizia, E., G. Andreucci, and D. Paolucci. 1979. Electroacupuncture and peripheral b-endorphin and ACTH levels. Lancet 2:535-536.
McCarthy, R.N., L.B. Jeffcott, and I.J. Clarke. 1993. Preliminary studies on the use of plasma b-endorphin in horses as an indicator of stress and pain. J. Equine Vet. Sci. 13:216-219.
Morley, J.E., A.S. Levine, G.K.W. Yim, and J.C. Liebsekind. 1985. Stress-induced analgesia in the mouse: Strain comparisons. Pain 23:67-72.
Nappi G., F. Fracchinetti, and G. Legnante. 1982. Different releasing effects on traditional manual acupuncture and electroacupuncture on proopiocortin-related peptides. Acupunct. Electro-ther. Res. 7:93-103.
Rubinstein, M., J.S. Mogil, M. Japon, E.C. Chan, R.G. Allen, and M.J. Low. 1996. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc. Nat. Acad. Sci. 93:3995-4000.

Figure 1. Average hormonal values for the period during exposure to the AI collection dummy compared to pre-exposure values. Bars marked with a letter superscript are different from the other treatment (a=P<.10). Asterisks indicate significant difference (**=P<.05) when compared to the pre-exposure period.
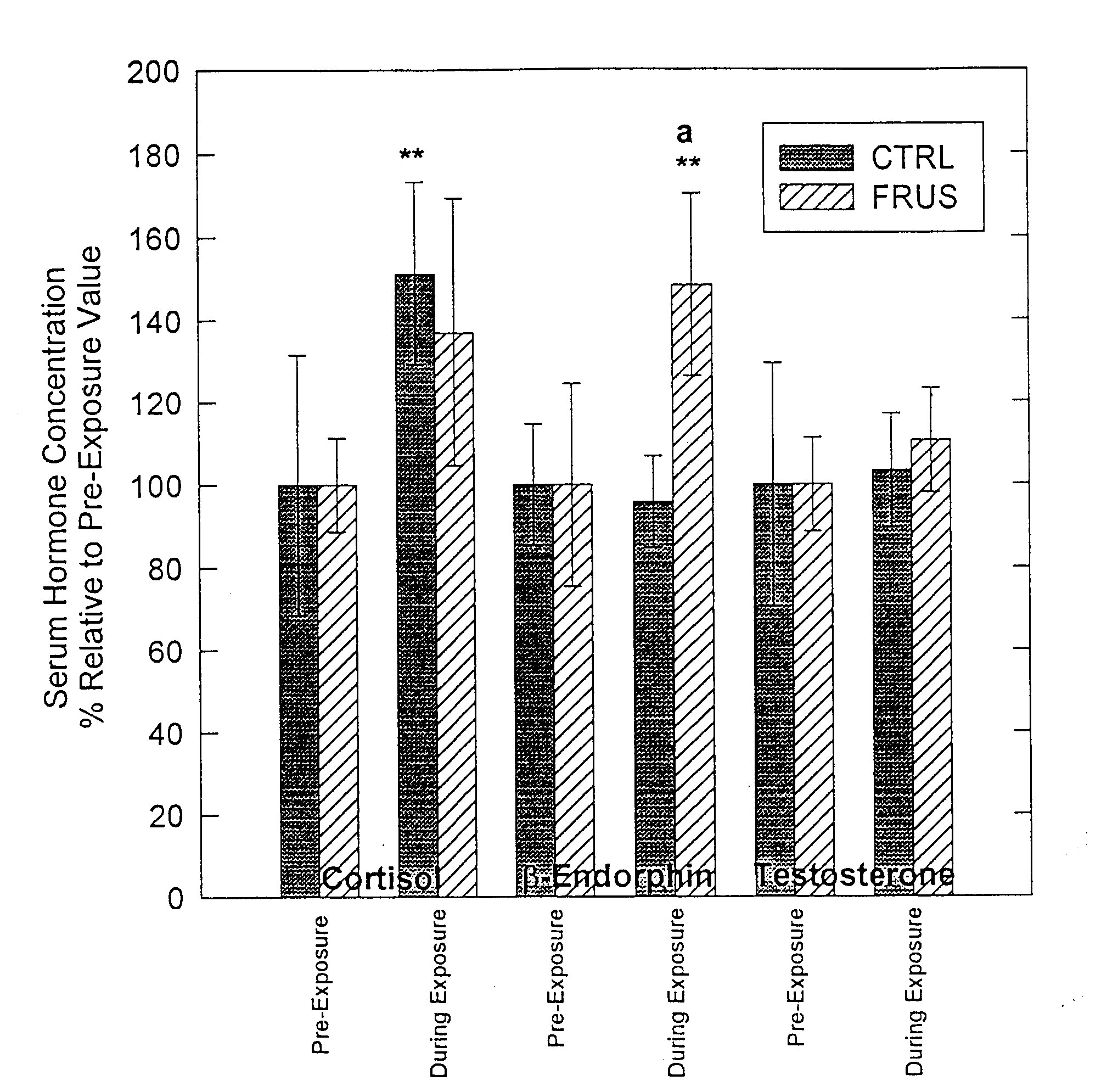
Figure 2. Average hormonal values for period after exposure to the AI collection dummy and return to the housing pen. Asterisks indicate significant difference (*=P<.10, **=P<.05) when compared to the pre-exposure period.
Index of 1997 Purdue Swine Day Articles
If you have trouble accessing this page because of a disability, please email anscweb@purdue.edu.