D.C. Kendall, B.T. Richert, T.E. Weber, K.A. Bowers, S.A.
DeCamp, A.P. Schinckel, and P. Matzat
Department of Animal Sciences and Roche Vitamins, Parker, CO
Introduction
Commercial swine production presents many challenges for producers because their pigs are often subject to environmental stressors that can limit their potential for growth. The stress level a pig experiences is a combination of physical stress (pen density, temperature and diet), social stress (mixing), and disease stress (health status and immune system activation) that is present in a herd. While physical and social stressors have been investigated extensively (Hyun et al., 1998), emphasis has only recently been focused on the effect that the health status of an animal has on its performance. Pigs reared in a commercial environment expressed only about 70% of the performance of those in unrestricted environments (Holck et al., 1998). Pigs with a high level of immune system activation had reduced feed intake and body weight gain, and different dietary amino acid requirements than those with a low level of immune system activation (Williams et al., 1997). It is well established that pigs from different genetic populations are variable in their growth performance and carcass characteristics (Schinckel and de Lange, 1996), but also in their response to different rearing environments (Kendall et al., 1999).
Feed grade antibiotics have been utilized as an effective means of improving growth rate and efficiency of growth in commercial environments. However, medicated diets have also been shown to have only modest or limited effect on growth rate in clean environments (Hays and Speer, 1960). The objectives of this study are to determine if differences exist in lean growth rate for pigs reared in different health status environments, and the effect that strategic antibiotic use has on performance in these two different health status environments.
Materials and Methods
Experimental design
Four hundred forty gilts, delivered from two different sources, were placed in all in/all out nurseries at 14 days of age (d14). Pigs were allotted by weight (10 pigs/pen) in a 2 x 2 factorial with two genotypes: Large White based x PIC C-22 (L) and Pietrain based x PIC C-22 (P), and two nursery dietary antibiotic treatments: medicated (NMED) and non-medicated diets (NNM). At 49 days of age, pigs were moved to either a continuous flow finisher (CF; N=192), in which every third pen consisted of older pigs, or an all in/all out finisher (AIAO; N=168) that had been previously cleaned and disinfected. Pigs were reallotted by weight (6 pigs/pen) into a 2 x 2 x 2 x 2 factorial composed of environment (ENV), genotype (GEN), prior nursery antibiotic treatment (NT) and finisher dietary antibiotic treatment (FT; medicated [FMED] or non-medicated [FNM]).
Diets
All diets were formulated to meet or exceed the requirement for all nutrients (NRC, 1998). Nursery diets were budget fed in four phases with medicated diets containing Denaguard Plus (400 g/ton CTC + 35g/ton tiamulin) in phase 1 and 250 g/ton ASP-250 in the remaining diets (Table 1). Finishing phase diets were fed in four phases, two grower diets and two finisher diets (Table 2), with diet changes at 100, 150, and 200 lbs of body weight (BW). Medicated grower diets contained 100 g/ton Aureomycin followed by finishing phase diets containing 50 g/ton Aureomycin.
Growth measurements
Pigs and feeders were weighed to determine average daily gain (ADG), average daily feed intake (ADFI) and gain:feed (G:F). Real-time ultrasound (Aloka 500) measurements were taken on 3 pigs/pen at days 49, 70, 104, 132 and 153 to determine loin eye area, and last rib and tenth rib backfat. Using this data, protein and lipid accretion curves were generated.
Carcass characteristics
Pigs were slaughtered at two time periods, either on day 153 or day 174, at a commercial slaughter facility. Carcass weight, backfat depth (BF), loin depth (LD) and fat-free lean index (FFLI) measurements were obtained from packer-prepared kill sheets of individual pigs.
Statistical analysis
Pen was the experimental unit for growth parameters and individual pig
was the experimental unit for carcass measurements. Growth and carcass
measurements were analyzed using the GLM procedures and are reported as least
squares means (SAS, 1996). Prediction equations by Bridges et al. (1986) were
used to fit weight to age using PROC NLIN of SAS (1996), ![]() , where WT is body weight minus birth weight (3 lbs), WTM is
an estimate of mature body weight, and T is days of age. Live weight, ultrasonic
tenth rib backfat depth and loin eye area were analyzed through a series of
prediction equations to estimate body composition (Wagner et al., 1999), and
data were fit to functions to determine total body fat-free lean mass (FFLA) and
total body fat mass (FA). FFLA was fit to a generalized non-linear function
, where WT is body weight minus birth weight (3 lbs), WTM is
an estimate of mature body weight, and T is days of age. Live weight, ultrasonic
tenth rib backfat depth and loin eye area were analyzed through a series of
prediction equations to estimate body composition (Wagner et al., 1999), and
data were fit to functions to determine total body fat-free lean mass (FFLA) and
total body fat mass (FA). FFLA was fit to a generalized non-linear function ![]() and FA was fit to an exponential function
and FA was fit to an exponential function
![]() .
.
The estimates of the lysine requirement (g/day) of pigs is based on energetic cost of lean accretion and an assumed maintenance requirement (Smith et al., 2000).
Results and Discussion
The growth performance of pigs during the nursery phase is listed in Table 3. For d14-28 and d28-42, the pigs receiving NMED diets had 11.7% higher ADG (P<.001) and 8.2% higher ADFI (d14-28, P<.05; d28-42, P<.01) than pigs fed NNM diets. These trends continued for the overall nursery period (d14-49), with the pigs fed NMED diets having 0.1 lb/day higher ADG, consuming 0.12 lb/day more feed (P<.001), and being 3.5 lbs heavier (P<.01) when leaving the nursery.
The pigs fed NMED diets were 2.8% more feed efficient during d14-28 (P<.05). This advantage disappeared over the remainder of the nursery phase, though a numerical improvement still existed. The variation in individual pig weight during the nursery phase is depicted in Table 4. The pigs fed NMED diets exhibited a trend for decreased variability by d28 and had significantly lower variation in pig weight by d42 (P<.05) than pigs fed the NNM diet. This increase in uniformity simplifies pig sorting, can increase the accuracy of diet changes and has potential implications on decreasing sort losses at market.
The growth performance in the finisher for pigs in each environment (ENV) are displayed in Tables 5 (CF) and 6 (AIAO). Data were separated into 5 periods - d49-90, d90-118, d118-153, d153-174 and overall - to better represent performance during periods of disease stress. The transition period from the nursery to the finisher is often stressful on pigs. In the CF environment, older pigs were placed in every third pen to enhance the transfer of disease to the incoming pigs. Much to our surprise, though, the pigs introduced into the CF environment exhibited performance equal to pigs in the AIAO environment in the early period of the finisher. However, pigs in the AIAO environment had 0.1, 0.45, and 0.34 lb/day higher ADG compared with pigs in the CF environment for d90-118 (P<.01), d118-153 (P<.001) and d153-174 (P<.001), respectively. The overall ADG (d49-market) for the finishing period was 11.8% higher for pigs in the AIAO environment compared to those in the CF environment. Pigs fed FMED diets had 3.1% higher ADG (P<.05) than those fed FNM diets for d49-90 and were numerically higher for all periods.
Pigs in the CF environment had 0.2 lb/day higher ADFI (P<.05) during d49-90 than those pigs in the AIAO environment. However, this trend was reversed for the remainder of the finishing phase and for the overall period, with the pigs in the AIAO environment consuming 6.1% more feed/day during the entire grow-finish period (P<.001). ADFI was 4.6% and 3.4% higher for pigs previously fed NMED diets during d49-90 and d90-118, respectively. Pigs receiving FMED diets also had 2.6% higher overall ADFI (P<.05). A three-way interaction of ENV by NT by FT occurred for overall ADFI (P<.05). Pigs in the CF environment fed both NMED and FMED diets had 0.33 lb/day higher intake than other treatment groups, while the other treatment groups were similar. However, in the AIAO environment, the pigs fed NMED and FMED diets were similar to pigs fed NMED-FNM or NNM-FMED diets, but the pigs fed NNM and FNM diets had 0.16 lb/day lower ADFI than the mean of other groups in the AIAO environment. Therefore, in a lower health environment, use of antibiotics improved ADFI to a greater extent than in a high health environment.
The pigs in the AIAO environment had 5.2%, 12.8%, and 5.3% higher G:F than pigs in the CF environment for d49-90, d118-153, and d49-market, respectively (P<.001).
Pigs in the AIAO environment were 12 lbs heavier and 6.5 days younger at slaughter than pigs in the CF environment (P<.001). This translated into a reduction in adjusted days to 250 lb (ADJ250) of 12 days for pigs from the AIAO environment compared to pigs in the CF environment. This is comparable to similar experiments where pigs from continuous flow environments were 11 days slower to 104 kg than those from all-in/all-out facilities (Cline et al., 1991). Pigs that were previously fed NMED diets were 3.5 lbs heavier entering the finisher and reached slaughter 3.8 days quicker than those previously fed NNM diets (P<.01). A three-way interaction of ENV by NT by FT occurred for ADJ250. Pigs in the CF environment receiving medicated diets in both phases were 8 days quicker to market weight than the mean of other groups in that environment. The pigs fed nursery antibiotic treatments in the AIAO environment did have a 4 day reduction in ADJ250 (P<.05), but there was no effect of finisher antibiotic in the AIAO environment on ADJ250. The ENV effect and interaction were expected, and it is reasonable to believe that if pigs are maintained in a high-health environment, their performance will be superior, and that differences in performance due to finisher feed-grade antibiotic treatments are more easily seen in environments with some level of disease pressure. The NT effect is interesting in that it shows the value of previous diet on future performance.
An ENV by FT interaction occurred for mortality, with the pigs in the CF environment fed FMED diets having 0.0% death loss and FNM fed pigs having 7.4% mortality, while both groups had 2.5% mortality in the AIAO environment. The percentage of pigs that were administered injectable medication (PIM) was higher in the CF environment than in the AIAO environment (44.5% vs. 8.1%; P<.001). The PIM values were also higher for pigs fed FNM diets in the finisher compared to those fed FMED diets (31.0% vs. 21.6%; P<.05). This was consistent in both environments (49.7 vs. 39.4% in the CF and 12.4 vs. 3.8% in the AIAO). A three-way and four-way interaction (P<.01) occurred due to both more consistent PIM values for the L pigs compared to the P pigs over the various treatment and environment combinations, and changes in order of treatment combinations for PIM in each environment and within genotype. The cost of one injection, in supplies and labor, will range from $0.60 to $0.90 depending on the cost of the antibiotic. Therefore, with an average of a 10 percentage point decrease in injectable antibiotic use seen for pigs fed Aureomycin in the finisher, the added cost of feed-grade antibiotics may be offset by decreasing death loss in sub-optimal environments and by decreasing the labor charges and expense of injectable medications, even in favorable environments.
Plant-measured carcass characteristics are shown in Table 7. A GEN by FT interaction occurred, with the L pigs fed FNM diets having 0.5% higher yield than the L pigs fed FMED diets, while the P genotype fed FMED diets had 0.3% higher yield than their FNM counterparts (P<.05). An NT by FT interaction occurred for backfat depth with the response to antibiotic inclusion in the diet being variable, with backfat depths ranging from 0.77 to 0.82 in. for the pigs fed NMED/FNM and NMED/FMED diets, respectively (P<.05), even though the differences are quite small. Also, a GEN by NT effect was seen, with the L pigs previously fed NMED diets having 0.04 in. less backfat than L pigs fed NNM diets, while P pigs previously fed NMED diets had 0.02 in. more backfat depth than P pigs fed NNM diets (P<.05). A small difference in muscle depth between the two genotypes was due to the P pigs having 0.06 in. more loin depth than L pigs (P<.05). There was a three-way interaction (ENV by NT by FT) for loin depth, with the pigs fed NMED-FMED diets and those fed NNM-FNM diets having the shallowest loin depths in the CF environment but having the largest values in the AIAO environment.
The plant FFLI mirrored the effects of fat depth, with significant interactions of NT by FT and GEN by NT. The NT by FT was being caused by a 0.6% difference in FFLI values for the NMED-FNM and NMED-FMED pigs (50.1% vs. 49.5%; P<.05). The GEN by NT interaction was the result of L pigs fed NMED diets having 0.5% higher FFLI and P pigs fed NMED diets having 0.3% lower FFLI values (P<.05).
The overall finishing period (d49-153) lean and fat accretion rates (Table 7) calculated from the ADG and real-time ultrasound data indicate that the pigs in the AIAO environment had 14% and 20% higher lean and fat accretion rates, respectively (P<.001). A GEN by ENV interaction occurred, with the L genotype having lower fat accretion in the CF environment and higher rates in the AIAO environment than the P genotype (P<.05). An NT by FT interaction occurred, with pigs fed NNM/FMED diets having the lowest fat accretion and the pigs fed NMED/FMED diets having the highest fat accretion (239 vs. 275 g/day [.526 vs. .606 lb/day]; P<.01). A three-way interaction of ENV by NT by FT occurred. Pigs in the AIAO environment ranged only 14 g/day (.031 lb) fat accretion for all treatment groups. However, in the CF environment, pigs fed NMED/FMED diets had 20 g/day (.044 lb) higher fat accretion rates than any other treatment group, while pigs fed NNM/FMED diets had the lowest fat accretion (P<.01).
The ADG curves are shown in Figures 1 (CF) and 2 (AIAO) for each environment and treatment group. In the CF environment, the pigs fed NMED/FMED diets exhibited the highest ADG. All pigs in this environment also had a steep decline in growth in the late finishing phase. Meanwhile, pigs in the AIAO environment had comparable growth rates among treatment groups and maintained a high level of performance throughout the grow-finish phase. Figures 3 (CF) and 4 (AIAO) are the lean accretion curves for each environment. The pigs in the CF environment reached their average peak lean accretion of 396 g/day (.872 lb) at a live weight of 87 lbs. In the AIAO environment, the average peak lean accretion was 373 g/day (.821 lb) at 127 lbs of live weight. The shapes of the curves for each environment also vary, with the pigs in the CF environment having a steep declining tail while those in the AIAO environment maintain relatively high lean accretion throughout the finishing phase.
Fat accretion is depicted in Figures 5 (CF) and 6 (AIAO). Rates of fat accretion are nearly identical in the AIAO environment between treatments and are comparable to those rates in the CF environment up to 158 lbs. However, in the CF environment, fat accretion reached a relative plateau at 185 lbs, while the pigs in the AIAO environment continued their linear increase in fat accretion throughout the finisher phase. Figures 7 (CF) and 8 (AIAO) show the estimated lysine requirement for pigs on trial based on their lean accretion and estimates of maintenance requirements. Pigs fed NMED/FMED diets in the CF environment had peak requirements 1.5 g/day higher than those fed NNM/FNM and NMED/FNM diets. All pigs in the AIAO environment had similar lysine requirements, but were 1.4 g/day higher than pigs in the CF environment from d49-153. From the growth curves, it is shown that the same genotype will exhibit vastly different lean and fat accretion rates depending on the rearing environment. Pigs in the AIAO environment attained their peak lean accretion 40 lb later than those pigs in the CF environment. In the AIAO environment, the nursery and finisher treatments did not affect the accretion curves. However, pigs that received both NMED and FMED diets did appear to have an advantage over other pigs in the CF environment in regards to ADG and lean accretion. This would imply that the lysine requirements (g/day) are increased when pigs are fed antibiotics in a poorer environment.
Conclusion
This trial would indicate that the use of Denaguard Plus followed by ASP-250 is effective at improving the performance of pigs in the nursery and can reduce variability in those pigs. Pigs receiving Aureomycin in the finishing phase have a reduced need for additional injectable medication and will exhibit lower death loss than pigs given no feed-grade antibiotics. It also demonstrated that an increase in disease pressure will result in changes in performance and carcass characteristics. These data support the prudent use of feed grade antibiotics, but for different reasons in different health status environments. Further research is required to enable researchers, nutritionists and producers to better adapt to changes in health status of pigs.
References
Bridges, T.C., L.W. Turner, E.M. Smith, T.S. Stahly, and O.J. Loewer. 1986. A mathematical procedure for estimating animal growth and body composition. Trans. ASAE. 29(5):1342-1347.
Cline, T.R., V.B. Mayrose, A.B. Scheidt, K. Clark, M. Diekman, C. Hurt, and W. Singleton. 1991. The effect of all-in/all-out management on the performance and health of growing-finishing pigs. Purdue University Swine Day Report. p. 1-4.
Hathaway, M.R., W.R. Dayton, M.E. White, and T.L. Henderson. 1996. Serum insulin-like growth factor concentrations are increased in pigs fed antimicrobials. J. Anim. Sci. 74:1541-1547.
Hays, V.W., and V.C. Speer. 1960. Effect of spiramycin on growth and feed utilization of young pigs. J. Anim. Sci. 19:938-942.
Holck, J.T., A.P. Schinckel, J.L. Coleman, V.M. Wilt, M.K. Senn, B.J. Thacker, E.L. Thacker and A.L. Grant. 1998. The influence of environment on the growth of commercial finishing pigs. Swine Health and Prod. 6:141-149.
Hyun, Y., M. Ellis, G. Riskowski, and R.W. Johnson. 1998. Growth performance of pigs subjected to multiple concurrent stressors. J. Anim. Sci. 76:721-727.
Kendall, D.C., B.T. Richert, J.W. Frank, S.A. DeCamp, B.A. Belstra, A.P. Schinckel, and M. Ellis. 1999. Evaluation of genotype, therapeutic antibiotic, and health-management effects and interactions on lean growth rate. Purdue University Swine Day Report. p. 75.
NRC. 1998. Nutrient requirements of swine (10th Rev. Ed.). National Academy Press, Washington, DC.
SAS. 1996. SAS/STATÒ User's Guide (Release 6.12). SAS Inst. Inc., Cary, NC.
Schinckel, A.P., and C.F.M. de Lange. 1996. Characterization of growth parameters needed as inputs for pig growth models. J. Anim. Sci. 74:2021-2036.
Smith, J.W., A.P. Schinckel, M.D. Tokach, S.S. Dritz, M. Einstein, J.L. Nelssen, and R.D. Goodband. 2000. The comparison of serial and mass growth and ultrasound composition assessments for the development of on-farm pig growth and composition curves. J. Anim. Sci. (submitted).
Wagner, J.R., A.P. Schinckel, W. Chen, J.C. Forrest, and B.L. Coe. 1999. Analysis of body composition changes of swine during growth and development. J. Anim. Sci. 77:1442-1466.
Williams, N.H., T.S. Stahly, and D.R. Zimmerman. 1997. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs from 6 to 112 kg. J. Anim. Sci. 75:2481-2496.
Table 1. Nursery diet composition.
|
Diet Composition |
Phase 1 |
Phase 2 |
Phase 3 |
Phase 4 |
|
Amount/pig fed or period |
3 lbs |
7 lbs |
15 lbs |
@d35-49 |
|
Crude Protein % |
|
|
20.9 |
21.3 |
|
Lysine % |
1.70 |
1.50 |
1.35 |
1.20 |
|
Tryptophan % |
|
0.32 |
0.27 |
0.27 |
|
Threonine % |
1.15 |
1.02 |
0.86 |
0.84 |
|
Met + Cys % |
0.95 |
0.84 |
0.76 |
0.72 |
|
Ca % |
0.85 |
0.85 |
0.90 |
0.85 |
|
P % |
0.75 |
0.75 |
0.80 |
0.75 |
|
|
|
|
|
|
|
Antibiotics |
Denagard Plus |
ASP-250 |
ASP-250 |
ASP-250 |
|
Medicated Diets (NMED) |
35g/ton timulin, |
250g/ton |
250g/ton |
250g/ton |
|
Control Diets (NNM) |
--- |
--- |
--- |
--- |
Table 2. Finisher diet composition.
|
Diet Composition |
Phase 1 |
Phase 2 |
Phase 3 |
Phase 4 |
|
Pig weight range (lbs) |
50-100 |
100-150 |
150-200 |
200-250 |
|
Crude Protein % |
18.4 |
17.3 |
15.2 |
13.1 |
|
Lysine % |
1.10 |
1.00 |
.85 |
.70 |
|
Tryptophan % |
0.24 |
0.22 |
0.15 |
0.15 |
|
Threonine % |
0.73 |
0.69 |
0.61 |
0.53 |
|
Met + Cys % |
0.63 |
0.61 |
0.56 |
0.51 |
|
Ca % |
0.75 |
0.75 |
0.70 |
0.65 |
|
P % |
0.65 |
0.65 |
0.60 |
0.55 |
|
|
|
|
|
|
|
Antibiotics |
Aureomycin |
Aureomycin |
Aureomycin |
Aureomycin |
|
Medicated Diets (FMED) |
100 g/ton |
100 g/ton |
50 g/ton |
50 g/ton |
|
Control Diets (FNM) |
--- |
--- |
--- |
--- |
Table 3. Effect of genotype and nursery treatment on nursery growth performance.
|
Genotypea |
L |
P |
|
|
||
|
Nursery Treatmentb |
Control |
Medicated |
Control |
Medicated |
CV |
Significancec |
|
ADG, lb/day |
||||||
|
Day 14-28 |
0.66 |
0.75 |
0.70 |
0.77 |
8.5 |
NT*** |
|
Day 28-42 |
1.10 |
1.25 |
1.11 |
1.22 |
8.3 |
NT*** |
|
Overall (d 14-49) |
0.94 |
1.04 |
0.95 |
1.05 |
5.9 |
NT*** |
|
ADFI, lb/day |
||||||
|
Day 14-28 |
0.76 |
0.83 |
0.82 |
0.88 |
10.0 |
NT* G* |
|
Day 28-42 |
1.70 |
1.86 |
1.73 |
1.85 |
7.8 |
NT** |
|
Overall (d 14-49) |
1.43 |
1.56 |
1.48 |
1.60 |
7.0 |
NT*** |
|
G:F |
||||||
|
Day 14-28 |
0.87 |
0.90 |
0.86 |
0.88 |
5.2 |
NT* |
|
Day 28-42 |
0.65 |
0.67 |
0.64 |
0.66 |
5.1 |
NT' |
|
Overall (d 14-49) |
0.69 |
0.69 |
0.67 |
0.68 |
4.7 |
--- |
|
Body weight, lb |
||||||
|
Initial (d 14) |
12.3 |
12.3 |
12.2 |
12.2 |
17.8 |
--- |
|
Final (d 49) |
45.9 |
49.5 |
46.3 |
49.8 |
7.2 |
NT** |
a
L = Large White based x PIC, P = Pietrain based x PIC.
Table 4. Effect of genotype and nursery treatment on nursery variability.
|
Genotypea |
L |
P |
|
||
|
Nursery Treatmentb |
Control |
Medicated |
Control |
Medicated |
Significancec |
|
Number of pigs |
110 |
110 |
108 |
110 |
|
|
Mean weight, lb |
|||||
|
Day 14 |
12.3 |
12.3 |
12.2 |
12.2 |
|
|
Day 28 |
21.6 |
22.8 |
22.0 |
23.1 |
|
|
Day 42 |
37.1 |
40.3 |
37.6 |
40.2 |
|
|
Standard Dev. |
|||||
|
Day 14 |
2.25 |
2.26 |
2.07 |
2.12 |
--- |
|
Day 28 |
3.61 |
3.22 |
3.33 |
3.05 |
NT' |
|
Day 42 |
5.96 |
5.06 |
4.98 |
4.53 |
NT* |
a
L = Large White based x PIC, P = Pietrain based x PIC.
Table 5. Effect of genotype, nursery and finisher treatment on growth performance in the continuous flow environment.
|
Environmenta |
CF |
|
|
|||||||
|
Genotypeb |
L |
P |
|
|
||||||
|
Nursery Treatmentc |
Control |
Medicated |
Control |
Medicated |
|
|
||||
|
Finisher Treatmentd |
Cont |
Med |
Cont |
Med |
Cont |
Med |
Cont |
Med |
C.V. |
Significancee |
|
Number of pens |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
|
|
|
ADG, lb/day |
||||||||||
|
Day 49-90 |
1.83 |
1.82 |
1.74 |
1.93 |
1.77 |
1.86 |
1.84 |
1.94 |
5.0 |
FT* NT' ExNTxFT' ExGxNT' |
|
Day 90-118 |
1.91 |
1.91 |
1.92 |
2.05 |
2.00 |
2.04 |
1.96 |
2.09 |
7.2 |
E** |
|
Day 118-153 |
1.50 |
1.39 |
1.38 |
1.50 |
1.60 |
1.45 |
1.56 |
1.54 |
9.0 |
E*** |
|
Day 153-174 |
1.48 |
1.53 |
1.44 |
1.91 |
1.51 |
1.67 |
1.63 |
1.70 |
16.0 |
E*** FT' |
|
Day 49-mkt |
1.71 |
1.67 |
1.65 |
1.81 |
1.74 |
1.74 |
1.76 |
1.81 |
4.3 |
E*** ExNTxFT' GxNTxFT' |
|
ADFI, lb/day |
||||||||||
|
Day 49-90 |
4.11 |
3.95 |
3.88 |
4.20 |
3.82 |
3.90 |
3.95 |
4.29 |
6.8 |
E** NT* ExNTxFT' |
|
Day 90-118 |
5.41 |
5.13 |
5.23 |
5.60 |
5.39 |
5.21 |
5.32 |
5.71 |
6.3 |
E* NT* ExNTxFT* |
|
Day 118-153 |
5.22 |
5.27 |
5.10 |
5.46 |
5.72 |
5.30 |
5.31 |
5.70 |
6.7 |
E*** ExNTxFT' ExGxNTxFT' |
|
Day 153-174 |
5.30 |
5.13 |
5.39 |
5.82 |
4.83 |
5.45 |
5.21 |
6.08 |
11.3 |
E*** FT* GxFT* |
|
Day 49-mkt |
4.87 |
4.76 |
4.75 |
5.06 |
4.89 |
4.82 |
4.80 |
5.23 |
4.8 |
E*** FT* NT' ExNTxFT* |
|
G:F |
||||||||||
|
Day 49-90 |
0.45 |
0.46 |
0.45 |
0.46 |
0.47 |
0.48 |
0.47 |
0.45 |
4.3 |
E*** GxE' NT' |
|
Day 90-118 |
0.35 |
0.37 |
0.36 |
0.36 |
0.37 |
0.39 |
0.37 |
0.37 |
3.9 |
E' NT' |
|
Day 118-153 |
0.28 |
0.25 |
0.26 |
0.27 |
0.27 |
0.27 |
0.29 |
0.23 |
9.5 |
E*** GxNTxFT' |
|
Day 153-174 |
0.28 |
0.30 |
0.26 |
0.33 |
0.31 |
0.31 |
0.31 |
0.28 |
12.6 |
--- |
|
Day 49-mkt |
0.35 |
0.35 |
0.35 |
0.36 |
0.36 |
0.36 |
0.37 |
0.35 |
3.6 |
E*** |
|
|
||||||||||
|
Initial wt., d49, lb |
48.8 |
46.3 |
48.3 |
50.7 |
47.0 |
46.5 |
48.4 |
50.6 |
8.9 |
NT** |
|
Off-test weightf, lb |
250.8 |
245.3 |
248.6 |
254.6 |
253.7 |
253.3 |
252.0 |
258.4 |
2.8 |
E*** |
|
Days of age |
167.4 |
168.5 |
170.5 |
161.6 |
167.7 |
167.7 |
164.8 |
163.7 |
3.0 |
E*** NT** |
|
Adj. days to 250g |
167.1 |
171.1 |
171.2 |
159.2 |
165.8 |
166.0 |
164.0 |
159.7 |
3.4 |
E*** NT* ExNTxFT* GxNTxFT' |
|
Death loss, % |
12.5 |
0 |
8.5 |
0 |
0 |
0 |
8.5 |
0 |
226 |
FT* ExFT* |
|
Morbidityh, % |
4.25 |
4.25 |
0 |
4.25 |
0 |
4.25 |
0 |
0 |
392 |
E' |
|
Percent of pigs adm. Inj. Med. |
62.3 |
33.1 |
41.4 |
54.0 |
29.1 |
49.8 |
66.3 |
20.7 |
60.7 |
E*** FT* GxNTxFT** ExGxNTxFT** |
a
CF = continuous flow.
Table 6. Effect of genotype, nursery and finisher treatment on growth performance in the all in/all out environment.
|
Environmenta |
AIAO |
|
|
|||||||
|
Genotypeb |
L |
P |
|
|
||||||
|
Nursery Treatmentc |
Control |
Medicated |
Control |
Medicated |
|
|
||||
|
Finisher Treatmentd |
Cont |
Med |
Cont |
Med |
Cont |
Med |
Cont |
Med |
C.V. |
Significancee |
|
Number of pens |
4 |
3 |
3 |
4 |
4 |
3 |
3 |
4 |
|
|
|
ADG, lb/day |
||||||||||
|
Day 49-90 |
1.73 |
1.80 |
1.88 |
1.87 |
1.84 |
1.88 |
1.86 |
1.85 |
5.0 |
FT* NT' ExNTxFT' ExGxNT' |
|
Day 90-118 |
2.04 |
2.06 |
2.14 |
2.07 |
2.09 |
2.11 |
2.13 |
2.07 |
7.2 |
E** |
|
Day 118-153 |
2.00 |
1.91 |
1.89 |
1.98 |
1.86 |
2.06 |
1.90 |
1.93 |
9.0 |
E*** |
|
Day 153-174 |
2.09 |
1.86 |
1.90 |
2.13 |
1.84 |
2.05 |
1.68 |
2.07 |
16.0 |
E*** FT' |
|
Day 49-mkt |
1.93 |
1.90 |
1.95 |
1.97 |
1.91 |
2.00 |
1.94 |
1.94 |
4.3 |
E*** ExNTxFT' GxNTxFT' |
|
ADFI, lb/day |
||||||||||
|
Day 49-90 |
3.58 |
3.62 |
3.86 |
3.94 |
3.71 |
3.91 |
3.97 |
3.92 |
6.8 |
E** NT* ExNTxFT' |
|
Day 90-118 |
5.42 |
5.41 |
5.76 |
5.58 |
5.49 |
5.56 |
5.77 |
5.54 |
6.3 |
E* NT* ExNTxFT* |
|
Day 118-153 |
6.40 |
6.29 |
6.42 |
6.58 |
6.05 |
6.72 |
6.40 |
6.44 |
6.7 |
E*** ExNTxFT' ExGxNTxFT' |
|
Day 153-174 |
6.42 |
6.18 |
6.58 |
6.43 |
6.46 |
7.19 |
5.30 |
6.90 |
11.3 |
E*** FT* GxFT* |
|
Day 49-mkt |
5.07 |
5.07 |
5.17 |
5.30 |
5.07 |
5.38 |
5.25 |
5.26 |
4.8 |
E*** FT* NT' ExNTxFT* |
|
G:F |
||||||||||
|
Day 49-90 |
0.49 |
0.50 |
0.49 |
0.48 |
0.50 |
0.48 |
0.47 |
0.48 |
4.3 |
E*** GxE' NT' |
|
Day 90-118 |
0.38 |
0.38 |
0.37 |
0.37 |
0.38 |
0.38 |
0.37 |
0.37 |
3.9 |
E' NT' |
|
Day 118-153 |
0.30 |
0.30 |
0.28 |
0.30 |
0.30 |
0.30 |
0.30 |
0.30 |
9.5 |
E*** GxNTxFT' |
|
Day 153-174 |
0.33 |
0.30 |
0.29 |
0.33 |
0.28 |
0.29 |
0.32 |
0.30 |
12.6 |
--- |
|
Day 49-mkt |
0.38 |
0.37 |
0.38 |
0.37 |
0.38 |
0.37 |
0.37 |
0.37 |
3.6 |
E*** |
|
|
||||||||||
|
Initial wt., d49, lb |
47.3 |
45.1 |
51.6 |
50.8 |
46.8 |
46.9 |
53.1 |
49.9 |
8.9 |
NT** |
|
Off-test weightf, lb |
264.2 |
266.5 |
260.1 |
264.7 |
263.4 |
269.5 |
261.3 |
263.9 |
2.8 |
E*** |
|
Days of age |
161.4 |
165.8 |
155.8 |
157.9 |
162.6 |
160.5 |
156.5 |
159.1 |
3.0 |
E*** NT** |
|
Adj. days to 250g |
155.0 |
158.2 |
151.3 |
151.4 |
156.4 |
151.9 |
151.5 |
152.8 |
3.4 |
E*** NT* ExNTxFT* GxNTxFT' |
|
Death loss, % |
4.25 |
0 |
5.6 |
4.25 |
0 |
5.6 |
0 |
0 |
226 |
FT* ExFT* |
|
Morbidityh, % |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
392 |
E' |
|
Percent of pigs adm. Inj. Med. |
4.2 |
11.1 |
11.0 |
4.2 |
12.4 |
0 |
22.0 |
0 |
|
E*** FT* GxNTxFT** ExGxNTxFT** |
a
AIAO = all in/all out.
Table 7. Effect of environment, genotype, nursery and finisher treatment on carcass characteristics and lean and fat accretion rates.
|
Environmenta |
CF |
|
|
|||||||
|
Genotypeb |
L |
P |
|
|
||||||
|
Nursery Treatmentc |
Control |
Medicated |
Control |
Medicated |
|
|
||||
|
Finisher Treatmentd |
Cont |
Med |
Cont |
Med |
Cont |
Med |
Cont |
Med |
C.V. |
Significancee |
|
Carcass meas. (N) |
18 |
20 |
20 |
21 |
23 |
22 |
20 |
23 |
|
|
|
Yield (%)f |
75.0 |
74.9 |
75.4 |
74.2 |
74.7 |
74.6 |
74.4 |
75.2 |
2.2 |
FTxG* |
|
BFfg, in. |
0.85 |
0.80 |
0.79 |
0.78 |
0.79 |
0.72 |
0.76 |
0.85 |
17.5 |
NTxFT* GxNT* ExFT' |
|
Loin depthf, in. |
2.12 |
2.14 |
2.12 |
2.08 |
2.08 |
2.16 |
2.20 |
2.09 |
9.9 |
G* ExNTxFT* ExG' |
|
FFLIf |
49.1 |
49.8 |
49.8 |
50.0 |
49.8 |
50.8 |
50.2 |
49.0 |
3.6 |
NTxFT* GxNT* ExFT' |
|
|
||||||||||
|
Fat accretion, lb/d |
.522 |
.420 |
.423 |
.568 |
.566 |
.480 |
.515 |
.605 |
18.6 |
E*** GxE* NT' NTxFT** ExNTxFT** |
|
Lean accretion, lb/d |
.692 |
.654 |
.643 |
.720 |
.696 |
.698 |
.687 |
.683 |
8.8 |
E*** GxNTxFT' |
|
|
||||||||||
|
|
||||||||||
|
Environmenta |
AIAO |
|
|
|||||||
|
Genotypeb |
L |
P |
|
|
||||||
|
Nursery Treatmentc |
Control |
Medicated |
Control |
Medicated |
|
|
||||
|
Finisher Treatmentd |
Cont |
Med |
Cont |
Med |
Cont |
Med |
Cont |
Med |
C.V. |
Significancee |
|
Carcass meas. (N) |
21 |
18 |
17 |
23 |
24 |
17 |
18 |
24 |
|
|
|
Yield (%)f |
74.6 |
74.5 |
74.9 |
74.3 |
74.7 |
75.1 |
75.0 |
75.2 |
2.2 |
FTxG* |
|
BFfg, in. |
0.85 |
0.83 |
0.78 |
0.82 |
0.77 |
0.83 |
0.75 |
0.83 |
17.5 |
NTxFT* GxNT* ExFT' |
|
Loin depthf, in. |
2.13 |
2.07 |
2.09 |
2.13 |
2.24 |
2.17 |
2.18 |
2.23 |
9.9 |
G* ExNTxFT* ExG' |
|
FFLIf |
49.0 |
49.3 |
50.0 |
49.5 |
50.1 |
49.4 |
50.3 |
49.3 |
3.6 |
NTxFT* GxNT* ExFT' |
|
|
||||||||||
|
Fat accretion, lb/d |
.599 |
.599 |
.630 |
.654 |
.610 |
.601 |
.632 |
.599 |
18.6 |
E*** GxE* NT' NTxFT** ExNTxFT** |
|
Lean accretion, lb/d |
.782 |
.766 |
.797 |
.791 |
.766 |
.777 |
.784 |
.777 |
8.8 |
E*** GxNTxFT' |
a
CF = continuous flow, AIAO = all in/all out.
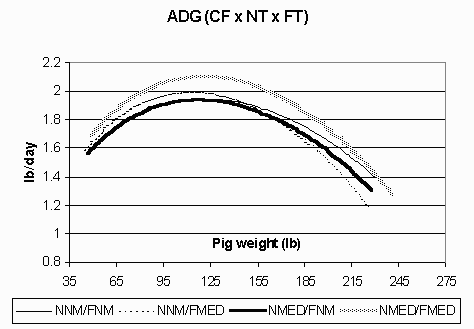
Figure 1. Average daily gain (lb/day; ADG) in the continuous flow (CF) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
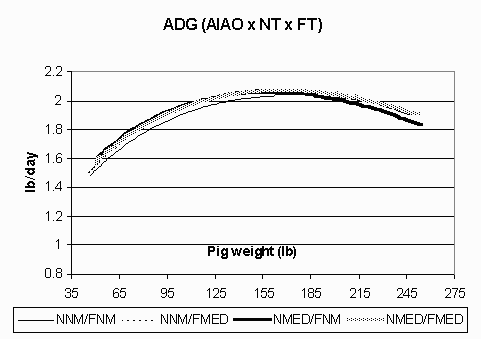
Figure 2. Average daily gain (lb/day; ADG) in the all in/all out (AIAO) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
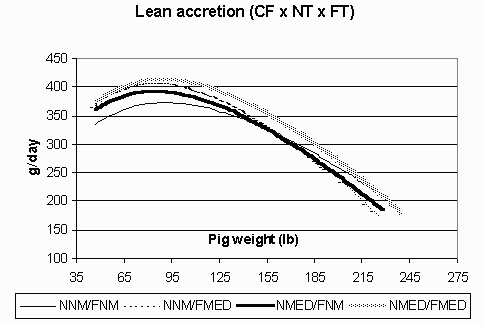
Figure 3. Lean accretion (g/day) in the continuous flow (CF) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
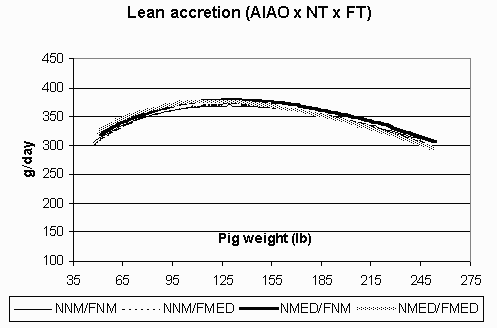
Figure 4. Lean accretion (g/day) in the all in/all out (AIAO) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
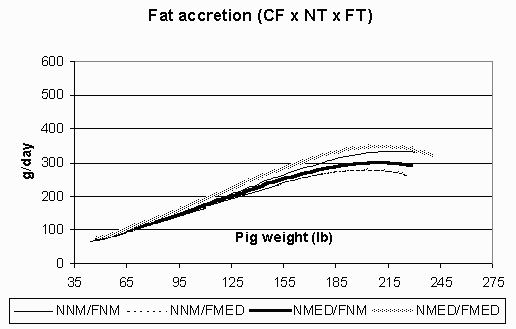
Figure 5. Fat accretion (g/day) in the continuous flow (CF) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
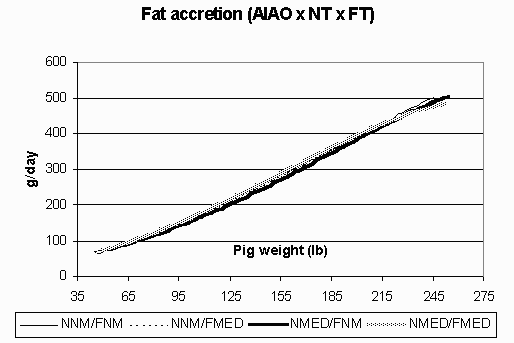
Figure 6. Fat accretion (g/day) in the all in/all out (AIAO) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
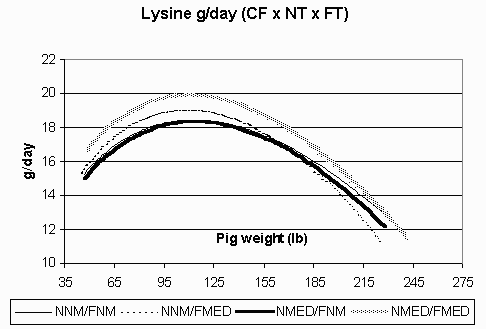
Figure 7. Estimated lysine requirement (g/day) in the continuous flow (CF) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).
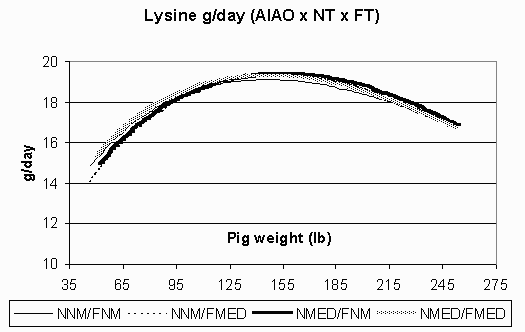
Figure 8. Estimated lysine requirement (g/day) in the all in/all out (AIAO) environment for pigs fed non-medicated diets in both the nursery and finisher (NNM/FNM), non-medicated diets in the nursery and medicated diets in the finisher (NNM/FMED), medicated diets in the nursery and non-medicated diets in the finisher (NMED/FNM), and medicated diets in both the nursery and finisher (NMED/FMED).